Description
The IMMUNO-TEK Goat IgG ELISA Kit is a rapid, easy to use enzyme linked immunosorbent assay (ELISA* ) designed for the measurement of goat IgG in goat colostrum, milk, serum, plasma or other biological fluids. The assay contains ready-to-use reagents and takes less than two hours to perform. The microplate and detector antibody in the kit have been specifically balanced to react uniformly with all subclasses of goat IgG.
* Research Purposes Only. Not For in vitro Diagnostic Use.
Zeptometrix | 0801223 | Goat IgG ELISA Datasheet
PRINCIPLE OF THE TEST
Microplate wells coated with polyclonal antibodies to goat IgG form the capture phase of the assay. These antibodies bind uniformly to all subclasses of goat IgG. Captured goat IgG then reacts with detector antibody, which is a polyclonal anti-goat IgG, conjugated with horseradish peroxidase. This reagent also reacts uniformly with all subclasses of goat IgG. Enzyme activity in the wells is then
quantified using tetramethyl benzidine as a substrate.
REAGENTS
Materials Supplied:
• Microplate, (1x96 well): Strips coated with purified rabbit anti-goat IgG
• Detector Antibody (12 ml): Contains conjugated rabbit anti-goat IgG peroxidase
• Goat IgG Standard (7 ml): Contains goat IgG and Assay Diluent
• Assay Diluent (100 ml): Contains PBS, Triton X-100® and 2-chloroacetamide
• Plate Wash Buffer (125 ml): Contains PBS, Tween 20® and 2-chloroacetamide
• Substrate (12 ml): Contains Tetramethyl Benzidine (TMB)
• Stop Solution (12 ml): Proprietary formulation
• Microplate Sealers (1 pk): 10 sealers per pack
• Plastic Bag (1 bag): For storage of unused microplate strips
® Triton X-100 is a registered trademark of Dow Chemical Company.
Tween 20 is a registered trademark of ICI Americas, Inc.
Materials required but not supplied:
• Personal protection equipment (Disposable gloves, lab coat, safety glasses, etc.)
• Distilled or deionized water
• Graduated cylinders and beakers
• Test tubes and racks for preparing specimen and IgG standard dilutions
• Validated adjustable micropipettes and tips, single and/or multi-channel
• Timer
• Validated incubator or temperature controlled room at 18-25˚C
• Absorbent paper towels
• Validated microplate reader capable of measuring optical density at 450nm
• Automatic microplate washer or manual vacuum aspiration equipment
• Graph paper or computer graphing software
STORAGE
Store all kit reagents at 2-8°C. Do not freeze. Unused microplate strips should be kept in a sealed bag with desiccant to minimize
exposure to moisture. All reagents stored and handled properly are stable at least until the expiration date printed on the kit box label.
PRECAUTIONS
• Prior to performing the assay, carefully read all instructions.
• Use universal precautions when handling kit components and test specimens.**
• To avoid cross-contamination, use separate pipette tips for each specimen.
• When testing potentially infectious specimens, adhere to all applicable local, state and federal regulations regarding the disposal of biohazardous materials.
• Stop Solution contains hydrochloric acid, which may cause severe burns. In case of contact with eyes or skin, rinse immediately with water and seek medical assistance. Wear protective clothing and eyewear.
**MMWR, June 24, 1988, Vol. 37, No. 24, pp. 377-382, 387-388.
PREPARATION OF REAGENTS
Plate Wash Buffer Dilute 10X Plate Wash Buffer 1:10 in distilled or deionized water prior to use. Mix thoroughly. Prepared 1X Plate Wash Buffer can be stored at 2-8°C for up to one week. Additional 10X Plate Wash Buffer (ZEPTOMETRIX Catalog #: 0801060) may be ordered if needed.
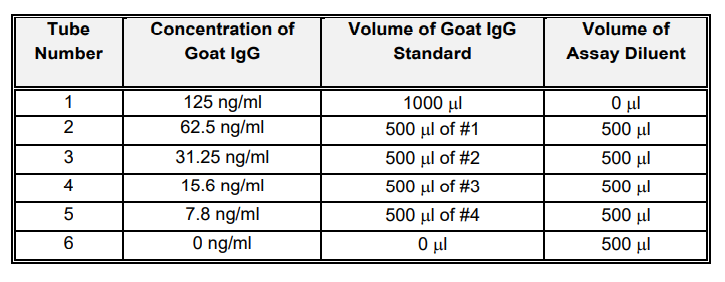
Goat IgG Standard Curve:
Label 6 test tubes as shown below. The Goat IgG Standard (125 ng/ml) should be diluted in Assay Diluent to prepare a standard curve as shown in the table below.
Specimen Dilutions:
Goat serum will normally contain ~20 mg/ml of IgG antibody. A 1:250,000-300,000 dilution of goat serum in Assay Diluent is recommended for initial testing. To reduce dilution error, three dilution steps are recommended. For example, three 1:65-fold serial dilutions, where 10ul is added to 640ul of Assay Diluent, is a convenient dilution scheme.
Goat IgG concentrations from other biological fluids will have to be estimated by the end-user to make the appropriate dilutions before sample testing. After initial testing, it may be necessary to adjust the dilution of the specimen sample in order to obtain an IgG concentration between 125 ng/ml and 7.8 ng/ml for accurate quantification.
TEST PROCEDURE
Allow all reagents to reach room temperature before use. Prepare reagents as described above. Label test tubes to be used for the preparation of standards and specimen samples. If the entire microplate will not be used for testing, remove surplus strips from the plate frame and place into the sealable plastic bag with desiccant. Seal bag and store at 2-8°C.
Step 1: Label each strip on its end tab to ensure identity should the strips become detached from the plate frame during the assay.
Step 2: Pipette 200 ul of standards 1-6 into duplicate wells.
Step 3: Pipette 200 ul of each specimen sample into duplicate wells.
Step 4: Cover the microplate with a plate sealer and incubate for 30 minutes at room temperature (18-25˚C).
Step 5: Aspirate the contents of each well and wash 4 times with 1X Plate Wash Buffer (See Preparation of Reagents section). To wash, fill the wells with at least 300 ul of 1X plate wash buffer and aspirate. Perform 4 fill/aspirate cycles. After the final wash cycle, thoroughly blot the plate by carefully striking on a pad of absorbent paper towels. Continue striking until no visible droplets of Plate Wash Buffer are observed.
Step 6: Pipette 100 ul of Goat IgG Detector Antibody into each well.
Step 7: Cover the plate with a plate sealer and incubate for 30 minutes at room temperature (18-25˚C).
Step 8: Wash the plate 4 times with 1X Plate Wash Buffer as described in Step 5.
Step 9: Pipette 100 ul of Substrate into each well.
Step 10:Incubate the plate for 30 minutes at room temperature (18-25˚C) without a plate cover. A blue color will develop in wells containing Goat IgG.
Step 11:Pipette 100 ul of Stop Solution into each well. A color change from blue to yellow will occur. Gently tap microplate to mix.
Step 12:Within 15 minutes, read the optical density of each well at 450 nm using a microplate reade
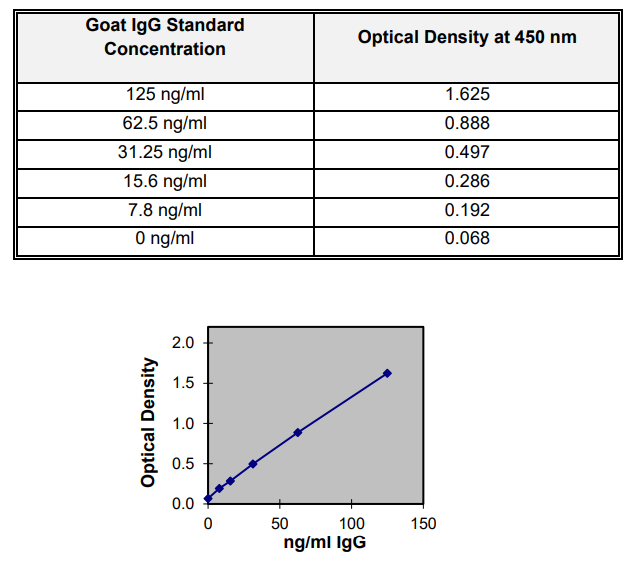
EXPECTED RESULTS
Below is an example of a standard curve and should not be used to calculate actual samples. Variations may be observed from laboratory to laboratory due to pipetting, incubator temperatures, plate readers, etc.
CALCULATION OF RESULTS
Test Validity:
• The mean optical density of the 0 ng/ml standard must be below 0.200.
• The mean optical density of the 125 ng/ml must be above 1.000.
• It is recommended that specimen samples be re-assayed with a greater or lesser dilution when the mean optical density value does not fall within the standard curve.
Calculation:
1. Calculate the mean optical density (OD) values for each set of standards and diluted specimen samples.
2. Using linear graph paper or graphing software, plot the mean OD values of each standard on the Y-axis versus the corresponding concentration of standards on the X-axis
3. Draw the best fit curve through these points to construct a standard curve. A point-to-point construction will be the most accurate.
4. Determine the IgG concentration of each diluted specimen sample by interpolation from the standard curve.
5. Multiply by the dilution factor used to dilute each specimen sample to determine the IgG concentration of the original specimen sample.
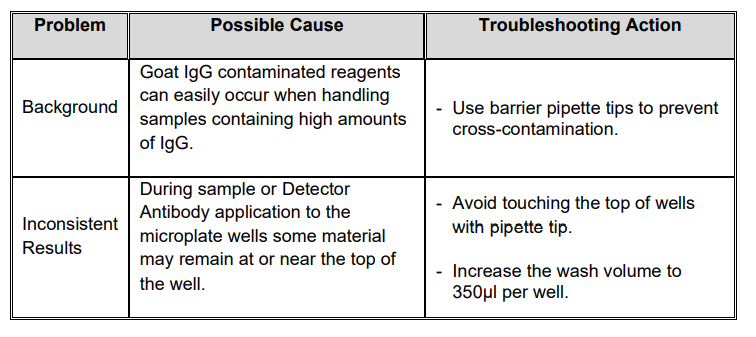
TROUBLESHOOTING
PROCEDURAL FLOW CHART
PREPARE REAGENT DILUTIONS
=>
PIPETTE SPECIMENS AND STANDARDS
=>
INCUBATE 30 MINUTES AT ROOM TEMPERATURE
=>
WASH PLATE
=>
PIPETTE DETECTOR ANTIBODY
=>
INCUBATE 30 MINUTES AT ROOM TEMPERATURE
=>
WASH PLATE
=>
PIPETTE SUBSTRATE
=>
INCUBATE 30 MINUTES AT ROOM TEMPERATURE
=>
ADD STOP SOLUTION AND READ AT 450 NM






