Description
Human Anti-Salmonella IgA Antibody Assay Kit - Cat Number: 6126 From .
Research Field: Bacterial Research, Immunology
Clonality: N/A
Cross-Reactivity:
Host Origin: N/A
Applications: N/A
Isotype: N/A
Detection Range: 32 units/ml-0.5units/ml
Sample Type: Serum, Plasma
Concentration: N/A
Immunogen:
PRODUCT SPECIFICATIONS
DESCRIPTION: ELISA kit to quantify mouse anti-bacteria and toxin IgG and IgA antibodies
FORMAT: Pre-coated 96-well ELISA Plate with removeable strips
ASSAY TYPE: Indirect ELISA
ASSAY TIME: 4.5 hours
STANDARD RANGE: 32 units/ml to 0.5 units/ml
NUMBER OF SAMPLES: Up to 40 (duplicate) diluted samples/plate and up to 20 (duplicate) low dilution samples/plate
SAMPLE TYPES: Serum and Plasma
RECOMMENDED SAMPLE DILUTIONS: 1:1000 (at least)
CHROMOGEN: TMB (read at 450 nm)
STORAGE: -20°C
VALIDATION DATA: 6113: Intra-Assay (3.2-7.2%)/Inter-Assay (3.7-6.5%)/Spiking Test (95-109%)
6114: Intra-Assay (3.3-8.8%)/Inter-Assay (4.2-9.8%)/Spiking Test (94-103%)
6115: Intra-Assay (1.2-4.7%)/Inter-Assay (6-9.8%)/Spiking Test (90-107%)
6116: Intra-Assay (1.1-5.3%)/Inter-Assay (2-10%)/Spiking Test (88-94%)
6117: Intra-Assay (2.5-8.8%)/Inter-Assay (5.8-9%)/Spiking Test (94-96%)
6118: Intra-Assay (1.1-8.9%)/Inter-Assay (4.5-9.9%)/Spiking Test (105-108%)
6119: Intra-Assay (1.9-7.3%)/Inter-Assay (6-9%)/Spiking Test (95-110%)
6120: Intra-Assay (3.3-9%)/Inter-Assay (1.5-10.9%)/Spiking Test (89-91%)
6121: Intra-Assay (1.3-7.3%)/Inter-Assay (4.4-9.2%)/Spiking Test (92-98%)
6122: Intra-Assay (0.6-6%)/Inter-Assay (6-9.4%)/Spiking Test (93-102%)
6123: Intra-Assay (1.2-8.9%)/Inter-Assay (1.7-8.9%)/Spiking Test (95-108%)
6124: Intra-Assay (3.5-7.3%)/Inter-Assay (4.3-9.7%)/Spiking Test (94-96%)
6125: Intra-Assay (4.2-8%)/Inter-Assay (4-7.8%)/Spiking Test (90-98%)
6126: Intra-Assay (2.9-4.9%)/Inter-Assay (4-8.4%)/Spiking Test (91-93%)
6127: Intra-Assay (1.9-7.5%)/Inter-Assay (6.5-8.3%)/Spiking Test (103-110%)
6128: Intra-Assay (5.8-9%)/Inter-Assay (3.4-9.8%)/Spiking Test (93-102%)
NOTES: N/A
INTRODUCTION
A growing body of research indicates an association between intestinal bacteria and autoimmune diseases. More specifically, dysbiosis or imbalance of intestinal bacterial flora may contribute to the pathogenesis of Rheumatoid Arthritis (RA), as indicated by many studies on intestinal microbes (1-5). Furthermore, recent studies have also suggested a possible link between RA and periodontal diseases caused by Porphyromonas gingivalis (P. gingivalis) and Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) (6-9). Normally, intestinal bacteria do not affect the host’s health, but under certain conditions, may overcome the host’s defenses and exert pathogenic effects. Examples of these include immunosenescence, gastrointestinal disorders such as constipation and diarrhea, or other events such as physical and psychological stress (10-17). It is therefore important to consider these risk factors when studying the etiology of autoimmune diseases. Intestinal bacterial imbalance may increase the levels of pathogenic substances in the gastrointestinal lumen and high mucosal permeability may increase translocation of potential pathogenic agents into the circulatory system. Consequently, pathogens can overwhelm the host’s defense functions and cause chronic health problems that can evolve into autoimmune disorders (18, 19). To facilitate and promote studies that determine immune responses to environmental agents in humans, , Inc. provides ELISA kits for assaying human serum antibodies against a variety of potential pathogenic and non-pathogenic environmental agents, of which all humans may be universally exposed to during their lifetime. For more information, please contact us at support@.com. These ELISA kits employ ChonBlock™ (Cat # 9068 and Cat # 90681) assay buffers. ChonBlock™ eliminates non-specific reactions involved in the indirect ELISA, especially false positive reactions caused by hydrophobic binding of immunoglobulins in sample specimens to ELISA plates as reported in detail (20-22).
PLATE COATING AND SETUP

KIT COMPONENTS
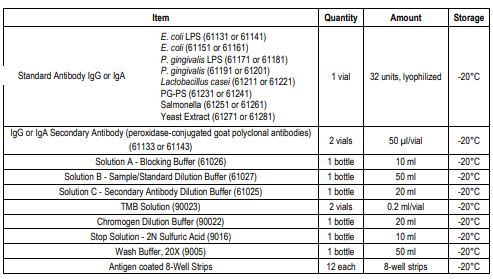
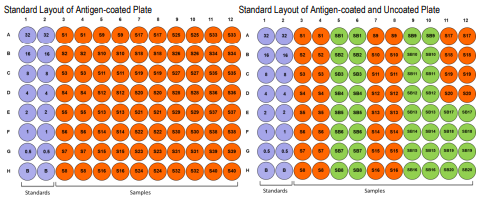
PLATE MAPPING
Map the plate based on the number of samples and sample dilution. For example, if sample dilution is more than 1:1,000, it is not necessary
to run antigen uncoated wells, but if sample dilution is less than 1:1,000, it is necessary to run antigen uncoated wells to determine the
background noise (BG) reaction OD values of individual samples.
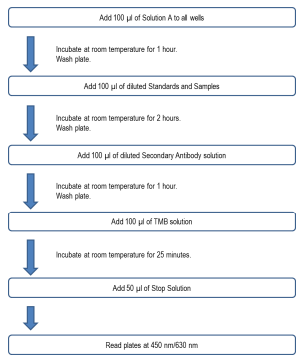
ASSAY OUTLINE
NOTES BEFORE USING ASSAY
NOTE 1: It is recommended that the standard and samples be run in duplicate.
NOTE 2: Warm up all buffers to room temperature before use.
NOTE 3: Crystals may form in Wash Buffer, 20X when stored at cold temperatures. If crystals have formed, warm the wash buffer by placing
the bottle in warm water until crystals are completely dissolved.
NOTE 4: Measure exact volume of buffers using a serological pipet, as extra buffer is provided.
NOTE 5: Cover the plate with plastic wrap or a plate sealer after each step to prevent evaporation from the outside wells of the plate.
NOTE 6: For partial reagent use, please see the assay protocol’s corresponding step for the appropriate dilution ratio. For example, if the
protocol dilutes 50 µl of a stock solution in 10 ml of buffer for 12 strips, then for 6 strips, dilute 25 µl of the stock solution in 5 ml of buffer.
Partially used stock reagents may be kept in their original vials and stored at -20⁰C for use in a future assay.
NOTE 7: This kit contains animal components from non-infectious animals and should be treated as potential biohazards in use and for
disposal.






