445
Mouse Anti-House Dust Mite (HDM) IgG3 Antibody Assay Kit
- SKU:
- 445-3039-GEN
- Availability:
- IN STOCK
Description
Mouse Anti-House Dust Mite (HDM) IgG3 Antibody Assay Kit - Cat Number: 3039 From .
Research Field: Allergy, Immunology
Clonality: N/A
Cross-Reactivity:
Host Origin: N/A
Applications: N/A
Isotype: N/A
Detection Range: 1000 ng/ml-16 ng/ml
Sample Type: Serum, Plasma
Concentration: N/A
Immunogen:
DESCRIPTION: ELISA kits to quantify mouse anti-house dust mite (HDM) antibodies
FORMAT: Precoated 96-well ELISA Plate with removeable strips
ASSAY TYPE: Indirect ELISA
ASSAY TIME: 4.5 hours
STANDARD RANGE: 3030 (IgG) : 100 - 1.6 ng/ml
3034 (IgG1) : 100 - 1.6 ng/ml
3035 (IgG2b) : 10 - 0.16 ng/ml
3036 (IgM) : 1000 - 16 ng/ml
3038 (IgG2a) : 50 - 0.8 ng/ml
3039 (IgG3) : 1000 - 16 ng/ml
3046 (IgA) : 500 - 8 ng/ml
NUMBER OF SAMPLES: Up to 40 (duplicate) samples/plate
SAMPLE TYPES: Serum & Plasma (pre-treatment acceptable)
RECOMMENDED SAMPLE DILUTIONS: 1:100 (at least)
CHROMOGEN: TMB (read at 450 nm)
STORAGE: -20°C for 12 months
VALIDATION DATA: 3030: Intra-Assay (5.5-9.6%)/Inter-Assay (2.9-7.0%)/Spiking Test (100-109%)
3034: Intra-Assay (1.7-5.4%)/Inter-Assay (3.6-12.6%)/Spiking Test (90-106%)
3035: Intra-Assay (5.8-10.1%)/Inter-Assay (3.1-9.8%)/Spiking Test (103-111%)
3036: Intra-Assay (3.1-8.3%)/Inter-Assay (0.9-4.8%)/Spiking Test (102-105%)
3038: Intra-Assay (2.7-6.3%)/Inter-Assay (7.4-11.5%)/Spiking Test (87-96%)
3039: Intra-Assay (1.5-7.2%)/Inter-Assay (1.5-9.3%)/Spiking Test (100-109%)
3046: Intra-Assay (2.4-7.7%)/Inter-Assay (7.8-10%)/Spiking Test (96-100%)
INTRODUCTION
Asthma is a common chronic inflammatory disease that affects 300 million people of all ages worldwide (1). It is caused by exposure to allergens such as dust mites, pet dander, pollen, or mold, and characterized by airflow obstruction and bronchospasm. House dust mite (HDM) is the most common asthma allergen, which affects up to 85% of asthma patients (2, 3). Of the two main mite species, Dermatophagoides pteronyssinus (Der p) and Dermatophagoides farinae (Der f), more than 20 types of HDM allergens are defined based on sequential and functional homologies. Among those HDM allergens, group 1 (Der 1) and group 2 (Der 2) dominate overall allergic responses in patients and are the most researched allergens (4-6).
Previously, asthma was considered an inflammatory airway disease mediated by the adaptive immune system, particularly type 2 helper Tcells (7).
However, recent studies indicate that the innate immune system is also involved in triggering an inflammatory response in both asthma patients and animal models (8-10). Airway remodeling and inflammatory changes significantly vary depending on the types of allergens (11). To meet such needs, a mouse HDM-induced asthma model is a useful tool to dissect the pathological roles of the adaptive and innate immune systems activated by the different HDM elements. This is an advantage over the classic ovalbumin-induced asthma model which activates adaptive immunity preferentially. Recently, it was reported that HDM-specific sublingual immunotherapy (SLIT) is more efficacious at preventing the development of allergic inflammatory reactions than subcutaneous immunotherapy in mouse models (12).
This SLIT protocol has been approved as a treatment to reduce allergy or asthma symptoms in patients (13).
To study the immune response to allergens and allergen-specific pathological effects in mouse models, , Inc. provides mouse antiHDM antibody ELISA kits listed below, including an anti-HDM IgE ELISA (Cat # 3037). , Inc. also offers ELISA kits for assaying anti-OVA antibody subtypes/subclasses as well as total immunoglobulin subtypes/subclasses. Please visit www..com for more information.
LIST OF MOUSE ANTI-HDM ANTIBODY SUBTYPE/SUBCLASS ELISA KITS
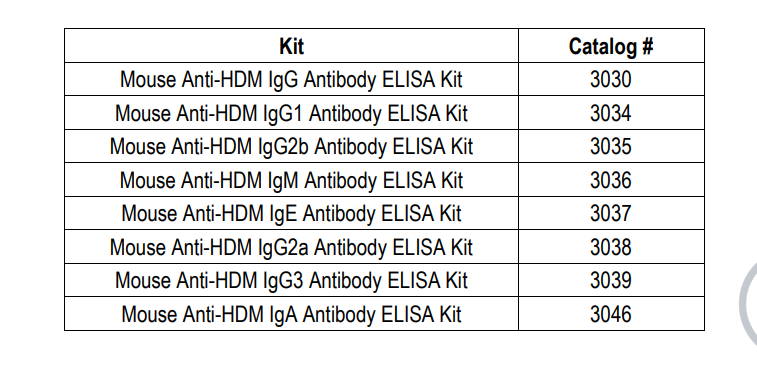
KIT COMPONENTS
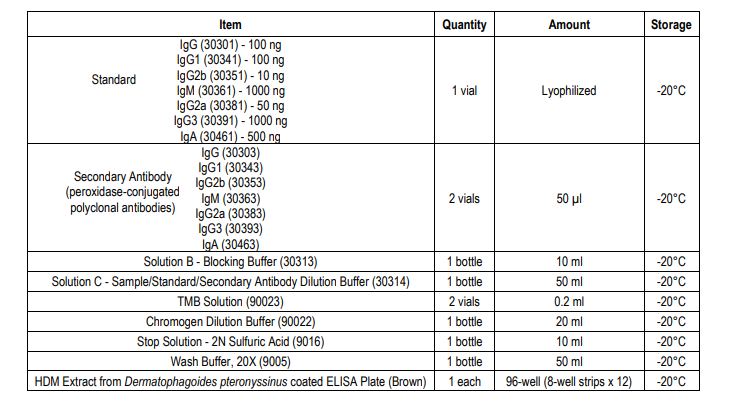
PLATE MAPPING
Example of the Mouse Anti-HDM IgG Antibody ELISA Kit
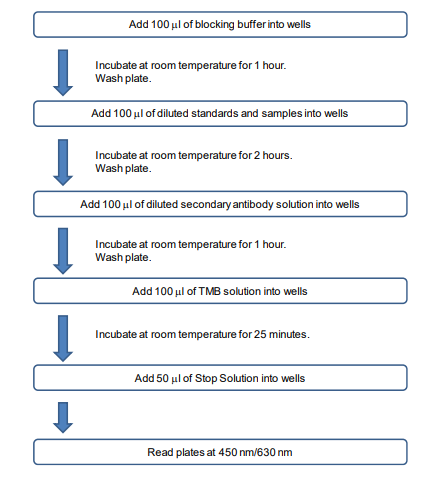
ASSAY OUTLINE
NOTES BEFORE USING ASSAY
NOTE 1: It is recommended that the standard and samples be run in duplicate.
NOTE 2: Warm up all buffers to room temperature before use.
NOTE 3: Crystals may form in Wash Buffer, 20X when stored at cold temperatures. If crystals have formed, warm the wash buffer by placing the bottle in warm water until crystals are completely dissolved.
NOTE 4: Measure exact volume of buffers using a serological pipet, as extra buffer is provided.
NOTE 5: Cover the plate with plastic wrap or a plate sealer after each step to prevent evaporation from the outside wells of the plate. NOTE 6: For partial reagent use, please see the assay protocol’s corresponding step for the appropriate dilution ratio. For example, if the protocol dilutes 50 µl of a stock solution in 10 ml of buffer for 12 strips, then for 6 strips, dilute 25 µl of the stock solution in 5 ml of buffer. Partially used stock reagents may be kept in their original vials and stored at -20⁰C for use in a future assay.
NOTE 7: This kit contains animal components from non-infectious animals and should be treated as potential biohazards in use and for disposal.
ASSAY PROCEDURE
1. Add Blocking Buffer: Add 100 µl of the Blocking Buffer (Solution B) to each well and incubate at room temperature for 1 hour.
2. Prepare Standard Dilutions: Please see the figure below for each assay’s recommended standard range. Dissolve one vial of Standard in 1 ml of Sample/Standard/Secondary Antibody Dilution Buffer (Solution C) and keep it as a standard stock. Then serially dilute it with Solution C. For example, mix 250 µl of the stock solution with an equal volume of Solution C to make the second stock solution, and then repeat it five more times. The remaining stock solution can be stored at -20⁰C for use in a future assay. , Inc. recommends making fresh serial dilutions for each assay.
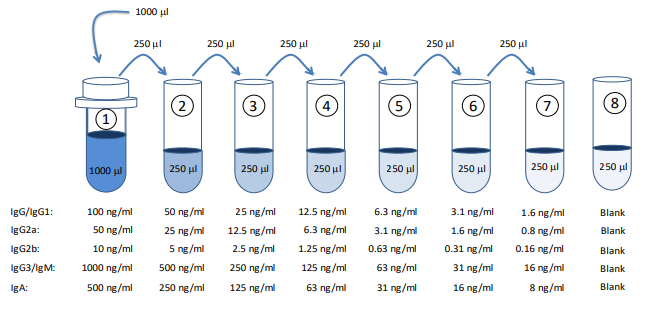
3. Prepare Sample Dilutions: The dilution of mouse serum immunized with HDM will vary (1:100 or more) depending on the immunization schedule and timing of serum collection. In general, no antibodies against HDM are observed in normal serum at a 1:100 dilution.
4. Wash: Dilute 50 ml of 20X wash buffer in 950 ml of distilled water (1X wash buffer). Wash the plate with 1X wash buffer at least 3 times using a wash bottle with manifold or an automated plate washer. Empty the plate by inverting it and blotting on a paper towel to remove excess liquid. Do not allow the plate to dry out.
5. Add Standards and Samples: Add 100 µl of standards, Solution C (blank), and samples to wells in duplicate. Incubate at room temperature for 2 hours.
6. Wash: Wash the plate with 1X wash buffer at least 3 times using a wash bottle with manifold or an automated plate washer. Empty the plate by inverting it and blotting on a paper towel to remove excess liquid. Do not allow the plate to dry out.
7. Add Secondary Antibody: Dilute one vial of Secondary Antibody in 10 ml Sample/Standard/Secondary Antibody Dilution Buffer (Solution C). Add 100 µl of secondary antibody solution to each well and incubate at room temperature for 1 hour.
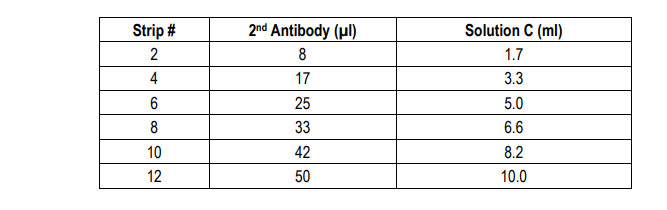
8. Wash: Wash the plate with 1X wash buffer at least 3 times using a wash bottle with manifold or an automated plate washer. Empty the plate by inverting it and blotting on a paper towel to remove excess liquid. Do not allow the plate to dry out.
9. Add TMB Solution: Use new tubes when preparing TMB solution. Just prior to use, prepare TMB solution with Chromogen Dilution Buffer as shown in the following table. Add 100 µl of TMB solution to each well immediately after washing the plate and incubate for 25 minutes at room temperature.
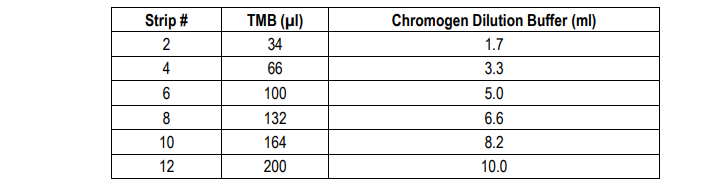
10. Stop: Stop the reaction with 50 µl of 2N Sulfuric Acid (Stop Solution) to each well.
11. Read Plate: Read the OD values at 450 nm. If the OD values of samples are greater than the OD values of the highest standard, reassay the samples at a higher dilution. A 630 nm filter can be used as a reference.
CALCULATING RESULTS
1. Average the duplicate OD values for the standards, blanks (B), and test samples.
2. Subtract the averaged blank OD values from the averaged OD values of the standards and test samples.
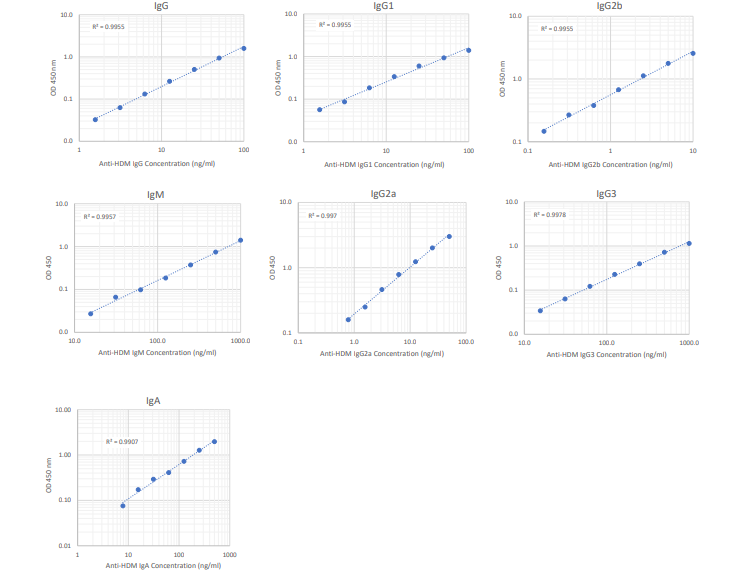
3. Plot the OD values of the standards against the ng/ml of antibody standard. Using a log/log plot will linearize the data. Figure 1 shows an example of a standard curve for anti-HDM IgG antibodies.
4. The ng/ml of antibody in test samples can be calculated using regression analysis. Multiply it by the sample dilution factor to obtain the antibody concentration (ng/ml) in original test samples.
Figure 1 - Typical Standard Curves for the Anti-HDM Antibody ELISA Kits