AMDX
AmoyDx® Pan Lung Cancer PCR Panel | 8.0131202W008
- SKU:
- AMDX-AM-8.0131202W008-GEN
- Availability:
- IN STOCK
Description
AmoyDx® Pan Lung Cancer PCR Panel | 8.0131202W008 | Gentaur UK, US & Europe Distribution
Lung cancer is one of the most common malignant tumor, and 80~85% of lung cancers are non-small cell lung cancer (NSCLC). There are many driver mutations in NSCLC. The frequency of mutations in NSCLC for EGFR, HER2, KRAS and BRAF genes are respectively 10-50%, 1-4%, 5-25% and 1-2%. About 3-7%, 1%, 1%, 0.12%, 0.02%, 0.08% of NSCLC patients have gene fusions in ALK, ROS1, RET, NTRK1, NTRK2 and NTRK3 genes, and approximately 1% of lung adenocarcinoma patients harbor MET exon 14 skipping mutations. Targeted therapies have been developed and approved for use in patients whose tumors have some of the genomic alterations seen in NSCLC. For instance, there are approved EGFR inhibitors, ALK inhibitors, ROS1 inhibitors, NTRK inhibitors and BRAF inhibitors for patients with specific genomic alterations in these genes. Testing for genomic alterations is a requirement in order to identify patients that may benefit from these targeted therapies and testing of multiple genomic alterations is recommended by the NCCN guidelines. Furthermore, there are many drugs in late stage development for other alterations (RET, MET, HER2 and KRAS)
Intended Use
The AmoyDx® Pan Lung Cancer PCR Panel is a real-time PCR assay for qualitative detection of 167 hotspot alterations in EGFR, ALK, ROS1, KRAS, BRAF, HER2, RET, MET, NTRK1, NTRK2 and NTRK3 genes. The kit is intended to be used to aid clinician to identify multigene status for NSCLC patients.
The kit is for in vitro diagnostic use, and intended to be used by trained professionals in a laboratory environment.
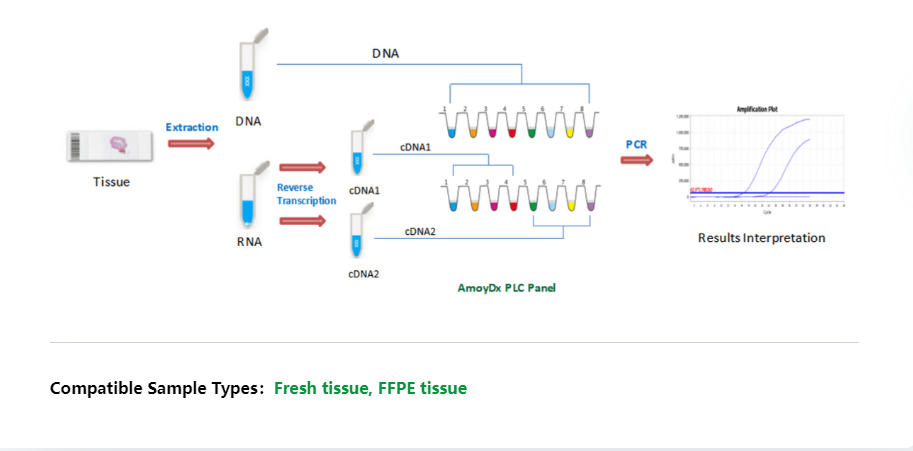
Additional Information
Status: |
CE Marked |
Type: |
Pre-loaded |
Group I: |
QuantStudio 5 |
Group II: |
LightCycler480 II |










