AMDX
AmoyDx® PIK3CA Mutation Detection Kit | 8.0121602X024E
- SKU:
- AMDX-AM-8.0121602X024E-GEN
- Availability:
- IN STOCK
Description
AmoyDx® PIK3CA Mutation Detection Kit | 8.0121602X024E | Gentaur UK, US & Europe Distribution
The phosphoinositide-3 -kinase catalytic alpha (PIK3CA) gene produces the p110 alpha (pl 10a) protein, which is one subunit of an enzyme called phosphatidylinositol 3-kinase (PI3K). PI3K plays a key role ofPI3K/Akt signaling pathway in numerous cellular processes critical for cancer progression, including metabolism, growth, survival, and motility. Somatic mutations in the PIK3CA gene are found in many types of cancer, including approximately 40% of patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer. PIK3CA mutations in which lead to Activation of the PI3K pathway in breast cancer have been typically associated with resistance to endocrine therapy and poor prognosis. The clinical studies demonstrate that PI3K inhibitors has shown significantly high response rate in patients with PIK3CA-mutated breast cancer.
Intended Use
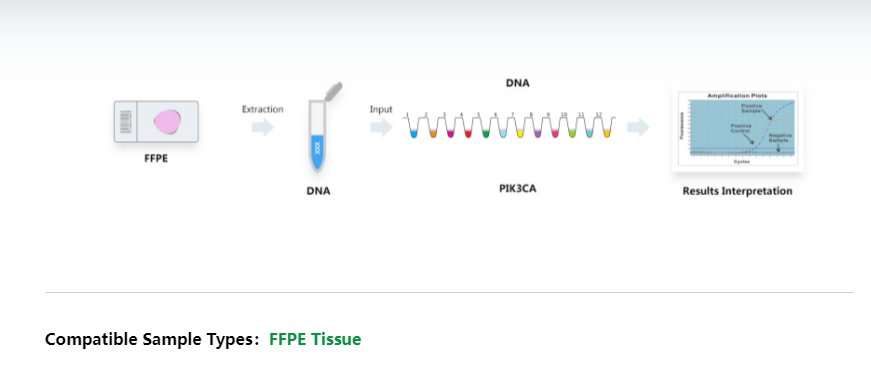
The AmoyDx® PIK3CA Mutation Detection Kit is a real-time PCR assay for qualitative detection of eleven mutations in the PIK3CA gene in human genomic DNA extracted from formalin-fixed paraffin-embedded (FFPE) tumor tissue.
The kit is for research use only, and intended to be used by trained professionals in a laboratory environment.
Principles of the Procedure
The kit adopts amplification refractory mutation system (ARMS) technology which comprises specific primers and fluorescent probes to detect gene mutations in real-time PCR assay. During the nucleic acid amplification, the targeted mutant DNA is matched with the bases at 3' end of the primer, amplified selectively and efficiently, then the mutant amplicon is detected by fluorescent probes labeled with FAM. While the wild-type DNA cannot be matched with specific primers, there is no amplification occurs.
Additional Information
Size: |
24 Tests/Kit |
Status: |
CE marked |
Type: |
Bulk |
PCR Instrument (stated in IFU): |
Stratagene Mx3000P™, ABI7500, LightCycler480, Bio-Rad CFX96, SLAN-96S |






