Description
Dengue genotype RT-PCR | V63-50FRT is available for delivery
Description:
General information: Real Time PCR kit for detection and differentiation of Dengue 1, 2, 3, 4
Target Disease Type: Dangerous Infections
Specific Application: Dengue Virus
Storage and Shipping : on request
Dengue genotype RT-PCR (CE) V63-50FRT DataSheet
INTRODUCTION
Dengue is one of the most important arthropod-borne viral diseases with large global burden. The disease is caused by dengue virus (DENV), a member of Flaviviridae family, with four distinct serotypes (DENV-1, -2, -3, and -4) circulating in tropical and subtropical regions in the world. DENV is transmitted to human by Aedes mosquitoes as vector. Dengue clinical manifestations vary from asymptomatic or mild flu-like syndrome known as classic Dengue Fever (DF) to more severe form known as Dengue Hemorrhagic Fever (DHF) and the potentially fatal Dengue Shock Syndrome (DSS). Similar to other RNA viruses, DENV possess diverse genetic characteristics as shown by the presence of various genotypes within serotypes.
There are four distinct dengue virus (DENV) serotypes that share antigenic relationships (DENV-1, DENV-2, DENV-3, and DENV-4), and although infection with one serotype confers lifelong protection against that serotype, it does not necessarily protect against a secondary infection with a heterologous serotype. Indeed, non protective but cross-reactive antibodies may enhance disease severity.
There are evidence that secondary infection with a heterologous DENV serotype, or primary infection in infants born to dengue-immune mothers, is an important individual risk factor for DHF/DSS. During secondary infection with a different serotype, the presence of low levels of heterotypic neutralizing antibodies may reduce disease severity. Alternatively, in the absence of such neutralizing antibodies, heterotypic cross-reactive antibodies may form complexes with the virus and the Fc-receptors on these complexed antibodies may attach to mononuclear phagocytes, thus enhancing the efficiency of infection and thereby increasing the number of infected mononuclear phagocytes. This phenomenon is known as antibody dependent enhancement (ADE).
The geographical areas in which dengue transmission occurs have expanded in recent years and all four dengue virus serotypes (DENV-1–4) are now circulating in Asia, Africa and the Americas, a dramatically different scenario from that which prevailed 30 or 40 years ago.
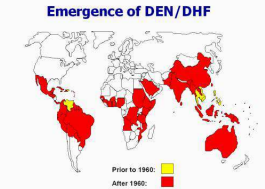
Estimates of the global incidence of dengue infections per year have ranged between 50 million and 200 million; however, recent estimates using cartographic approaches suggest this number is closer to almost 400 million.
The expansion of dengue is expected to increase due to factors such as the modern dynamics of climate change, globalization, travel, trade, socioeconomics, settlement and also viral evolution. No vaccine or specific antiviral therapy currently exists to address the growing threat of dengue.
Acute infection with dengue virus is confirmed when the virus is isolated from serum or autopsy tissue specimens, or the specific dengue virus genome is identified by reverse transcription[1]polymerase chain reaction (RT–PCR) from serum or plasma, cerebrospinal fluid, or autopsy tissue specimens during an acute febrile illness. Methods such as one-step, real time RT–PCR are now widely used to detect dengue viral genes in acute-phase serum samples, approximately the first 5 days of symptoms. This detection coincides with the viremia and the febrile phase of illness onset.
INTENDED USE
DENGUE Real-TM Genotype PCR kit is an in vitro nucleic acid amplification test for detection and differentiation of RNA of Dengue virus type 1-4 in the clinical materials (blood plasma, blood serum) and in the human’s autopsy materials (brain, liver, spleen tissues), in animal’s materials (brain, spleen tissues), in mosquitoes by using real-time hybridization-fluorescence detection of amplified products.
PRINCIPLE OF ASSAY
Dengue virus detection includes RNA isolation from biological materials and reverse transcription of RNA into cDNA combined with real-time PCR amplification of cDNA. Dengue detection by the polymerase chain reaction (PCR) is based on the amplification of pathogen genome specific region using specific Dengue genotype primers. In real-time PCR, the amplified product is detected using fluorescent dyes. These dyes are linked to oligonucleotide probes which bind specifically to the amplified product. The real-time monitoring of fluorescence intensities during the real-time PCR allows detection of the amplified product without re-opening the reaction tubes after the PCR run.
Dengue Real-TM Genotype PCR kit uses “hot-start”, which greatly reduces the frequency of nonspecifically primed reactions.
- The DENGUE genotype 1 cDNA is detected in the FAM/Green channel.
- The DENGUE genotype 2 cDNA is detected in the JOE/HEX/Yellow channel
- The DENGUE genotype 3 cDNA is detected in the Rox/Texas Red/Orange channel.
- The DENGUE genotype 4 cDNA is detected in the Cy5/Red channel
- The signal of the IC is detected in the Cy5.5/Crimson/ Quasar705 channel*
*only for 5-channels Real Time PCR instruments. On 4-channels RT PCR instrument kit can be used without IC detection.
The Positive Control of Amplification, Positive Control DV 1-4 types/IC is detected in FAM/Green (genotype 1), JOE/HEX/Yellow (genotype 2), ROX/Texas Red/Orange (genotype 3), Cy5/Red (genotypes 4) and Cy5.5/Crimson/ Quasar705 (Internal Control) channels.






