Gentaur
Monkeypox Virus Nuclei Acid Detection Kit (Fluorescence PCR Method) | GEN-MPV-04
- SKU:
- GEN-MPV-04-GEN
- Availability:
- IN STOCK
Description
Monkeypox Virus Nuclei Acid Detection Kit (Fluorescence PCR Method) | yY22PCR096 | GEN-MPV-04
Intended Use
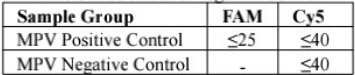
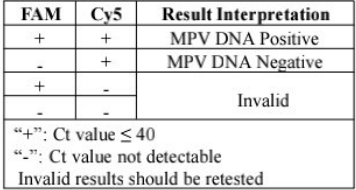
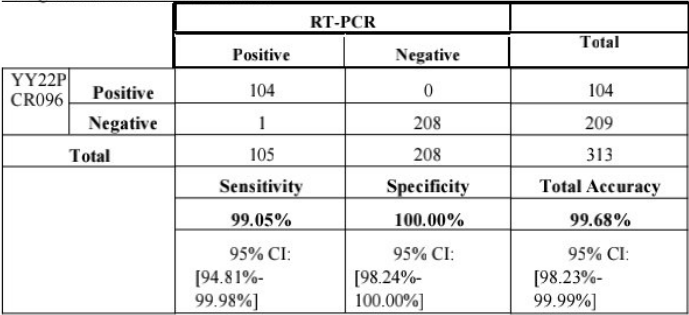
This kit is intended for the qualitative detection of monkeypox virus (MPV) in serum or lesion secretions. Detection results can be used to assist clinical diagnosis of monkeypox.The monkeypox virus is the causative pathogen of monkeypox, which is a viral zoonotie disease with symptoms in humans similar to those seen in smallpox patients. Farly symptoms include lever, headache, muscle pains, fatigue, and swollen glands. After a few days of carly symptoms, patients may develop a few to thousands of characteristic lesions on the face hands, and feet The monkeypox virus can be lound in the patients hody fluid and contaminated belongings, and spread by close contact with contaminated items.
Principle
- Read the package insert carefully prior to first use.
- The Kit should be used by PROFESSIONAL PERSONNEL ONLY.
- Wear personal protective equipment (PPE) before handling materials with potential biohazard risks.
- A void repeated thawing and freezing of product components. Once the components is thawed, vortex and centrifuge briefly before use.
- Dispose of used products, pipette tips, and other consumables as bio-hazard wastes. Do not reuse them.
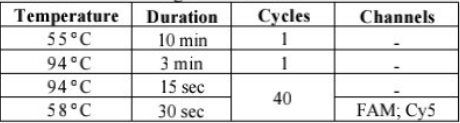
TEST PROCEDURES
Read the Package Insert Carefully Prior to First Use. Fquipment and Instruments Required but Not Provided in This Product
1. Real time PCR system
2. Real time PCR reaction tubes/plates
3. Viral DNA extraction kit
4. A Vortex mixer
5. High-speed centrifuge
6. Refrigerator and freezer
7. Pipettes and pipelte lips X Disposable gloves Q Safety goggles