Monkeypox Virus Real Time PCR Test Kit | GEN-MPV-02
Intended Use
The Monkeypox Virus Real Time PCR Test Kit is a real-time (rt) polymerase chain reaction (PCR) test intended for the qualitative detection of nucleic acid from monkeypox in skin lesion exudate swabs, lesion roofs and lesion crusts material collected at a healthcare location or collected by a healthcare provider, from individuals suspected of monkeypox infection by their healthcare provider. Testing is limited to professional use. Results are for the identification of Monkeypox virus DNA. The Monkeypox virus DNA is generally detectable in skin lesion specimens during the acute phase of infection. Positive results are indicative of the presence of Monkeypox virus DNA; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co infection with other viruses. Negative results do not preclude Monkeypox virus infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information. The Monkeypox Virus Real Time PCR Test Kit is intended for use by qualified and trained clinical laboratory personnel specifically instructed and trained in the techniques of real-time PR and in vitro diagnostic procedures. For in vitro diagnostic use only.
Method
The Monkeypox Virus Real Time PCR Test Kit is based on fluorescent modification of the PCR method. The PCR-mix contains target-specific probes bearing reporter fluorescent dyes and quencher molecules. Once hybridized to a target sequence, the probes become activated. As a result of activation fluorescence increases proportionally to target sequence amplification. The intensity of fluorescence is measured at every cycle of reaction with a Real- time PR thermal cycler data collection unit and analyzed with the software provided. The PCR-mix includes the Internal control (IC), which is intended to assess the quality of the polymerase chain reaction. DNA probes used for the detection of the Monkeypox Virus product amplification include fluorescent dyes Fam and Cy5. DNA probe used for the detection of the internal control amplification product includes the fluorescent dye Hex.

Reagents
1. Monkeypox Virus Internal Control (4 vials, 1.2mL per vial)
2. PCR Amplification Butfer (96 tests) Include synthetic oligonucleotides(primers and probes), dNTPs in a buffered solution with a reference dye. Preservative: 0.10% ProClin 300 and 0.15% ProClin 950.
3. Taq-polymerase solution(1 vial, 0.15mL per vial) 3.0 Units/uL in buffered solution
4. Monkeypox Virus Positive Control (8 vials, 1.5mL per vial)
5. Monkeypox Virus Negative Control (8 vials, 1.5mL per vial)
All components are ready to use and do not require additional preparation for operation. The Monkeypox Virus Real Time PCR Test Kit is intended for single use and designed for 96 tests.
Transport And Storage Condition
Expiry date - 12 months from the date of production. All components of the Monkeypox Virus Real Time PCR Test Kit must be stored at temperatures from -20°C=3°C, keep away from exposure to sunlight. Transportation is allowed in thermal containers with icepacks by all types of covered transport at temperatures from 2 °C to 8 °C inside the container over the transportation or at a temperature from 2 °C to 25 °C inside the container, but for no longer than 5 days.
Warning And Precausion
Only personnel trained in the methods of molecular diagnostics and the rules of work in the clinical and diagnostic laboratory are allowed to work with the kit. Handle and dispose all biological samples, reagents and materials used to carry out the assay as if they were able to transmit infective agents. The samples must be exclusively employed for certain type of analysis. Samples must be handled under a laminar flow hood. Tubes containing different samples must never be opened at the same time. Pipettes used to handle samples must be exclusively employed for this specific purpose. The pipettes must be of the positive dispensation type or be used with aerosol filter tips.The tips employed must be sterile, free from the DNases and RNases, free from DNA and RNA. Avoid direct contact with the biological samples reagents and materials used to carry out the assay. Use powder-free surgical gloves. Use protective clothing (work clothes and personal protective equipment) working with microorganisms classified as particularly pathogenic. The protective clothing and personal protective equipment must comply with the work to be performed and health and safety requirements. Avoid producing spills or aerosol. Any material being exposed to biological samples must be treated for at least 30 minutes with disinfecting solution or autoclaved for 1 hour at 121 °C before disposal. Molecular biology procedures, such as nucleic acids extraction, reverse transcription, amplification and detection require qualified staff to avoid the risk of erroneous results, especially due to the degradation of nuclei acids contained in the samples or sample contamination by amplification products. All oligonucleotide components are produced by artificial synthesis technology according to internal quality control protocol and do not contain blood or products of blood processing. Positive control is produced by artificial DNA synthesis technology. Positive control does not include parts of infectious agents.
Specimen Collection And Handling
The Monkeypox Virus Real Time PCR Test Kit is designed to detect DNA extracted from the skin lesion material, including swabs of lesion exudate, lesion roofs, lesion crusts, or other specimen depending on professional prescription. Sampling, sample processing procedures and storage are carried out in accordance with the instructions to the DNA extraction kit from biological material.
Test Procudues
DNA extraction is carried out according to the extraction kit instructions. PrePSEQTM 1-2-3 Nucleic acid extraction kit is recommended. It is allowed to use any kits of reagents registered as a medical device and recommended by manufacturers for the extraction of DNA from the corresponding types of biomaterial.
Assay Procedure
Step 1 :
Mark the required number of strips with paraffin sealed PCR-mix for each test sample, positive control (C+) and negative control (C-).
Step 2:
Vortex the MAX Taq-polymerase solution for 3-5 seconds, then spin for 1-3 seconds to collect the drops.
Step 3:
Add 10uL of MAX Taq-polymerase solution into each tube. Avoid paraffin layer break.
Step 4:
Add one drop (about 20 pL) of mineral oil into each tube of the strip. Close strips tightly.
Step 5:
Vortex the tubes with samples, "C+" and "C-" and for 3-5 seconds and spin down drops for 1-3 seconds.
Step 6:
Add 5.0 uL of DNA sample into corresponding strips. Do not add DNA into the "C+", "C-" strips. Avoid paraffin layer break.
Step 7:
Add 5.0 uL of negative control (C-) which passed whole DNA extraction procedure into corresponding tubes of the strip. Add 5.0 pL of positive control sample (C+) into corresponding tubes of the strip. Avoid paraffin layer break.
Step 8:
Spin strips for 3-5 seconds.
Step 9:
Set the strips into the Real-time Thermal Cycler.
Step 10:
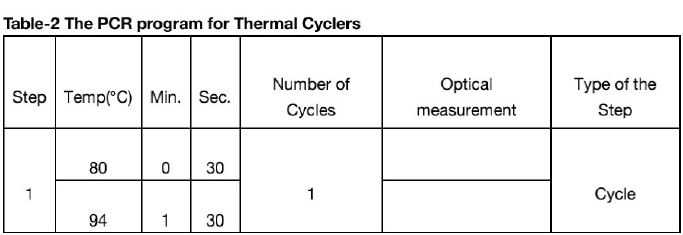
Launch the Real Time PR application in "Device operation" mode. Upload the ini file supplied with the kit before first run. Please refer to thermal cycler's user manual for details on working with ini files. In subsequent runs add corresponding test to the protocol, specify the number and ID's of the samples, specify the position of the tubes in the thermal unit and run PCR.
Controls
The Monkeypox Virus Real Time PCR Test Kit contains positive control sample. Positive control is a cloned part of the monkeypox genome. It is produced with genetic engineering techniques and characterized by automatic DNA sequencing. The PCR-mix from the kit includes the Internal control (IC). IC is an artificial plasmid intended to assess the quality of PR performance. To reveal possible contamination a negative control is required. The test result is considered valid when: - Positive result for the specific product is present, in this case the internal control is not taken into account. In the presence of monkey pox virus DNA in the test sample, the absolute quantity of this virus type (the degree of concentration common logarithm, number of copies of the monkeypox virus DNA per sample) will be specified in the line with the name of this type of monkeypox virus in the "Quantitative" field (absolute analysis). - Positive result for the specific product is absent and for internal control is present. The test result is considered invalid when a positive result for the specific product and for internal control is absent. If positive control (C+) has not positive result for the specific product, it is necessary to repeat the whole test. It may be caused by inhibitors, operation error or violation of storage and handling requirements.
Data Analysis
Registration and interpretation of the PR results are held in automatic mode. Analysis will be performed by Real-Time PCR application. The resulting graph will display the dependence of fluorescence intensity on the cycle number for each tube. Type of the sample, name of the test, value of the threshold cycle (Cp) and test result (relative, absolute and quantitative) will be displayed in the right module of the window. Operator can create, save and print a report.
Specification
a. The analytical specificity of the Monkeypox Virus Real Time PCR Test Kit was assessed by bioinformatics analysis using available online databases with up-to-date comprehensive genetic information. The specific oligonucleotides used in the test were checked against GenBank database sequences. None of the sequences showed sufficient similarity for unspecific detection. The samples with monkeypox virus DNA are to be registered positive for specific product (a fragment of the monkeypox virus genome). The samples free of monkeypox virus DNA are to be registered negative for specific product and positive for internal control. There are not non-specific positive results of amplification of DNA sample in the presence of Mycoplasma dentalium, Mycoplasma hominis, Chlamydia trachomatis, Candida albicans, Streptococcus sp., Staphylococcus sp., Lactobacillus spp., EBV.
b. In a determination of analytical sensitivity, The onkeypox Virus Real Time PCR Test Kit demonstrated the ability to reproducibly detect 5 or more copies of purified pathogens DNA per PCR reaction (103 copies/mL DNA sample). The virus copies' number was determined by Poison analysis. The Monkeypox Virus Real Time PCR Test Kit detects one FU of the pathogen per PR reaction. This analytical sensitivity was determined by serially diluting pathogens infected cultures in culture transport media. Samples of each dilution were processed and tested by the standard Kit procedure. Each of the replicates containing 1 CFU per amplification reaction gave a strong positive signal. The analytical sensitivity depends on the type of biomaterial, DNA extraction kit and the final volume of extracted DNA elution. For example: the analytical sensitivity of the kit is 600 copies/sample when DNA is extracted from a sample Extraction Kits (elution volume is 300 HIL).
c. Sample Intake Control
During amplification of biological samples containing human genomic DNA the Real-Time PCR instrument should record the exponential growth of the fluorescence level in the corresponding tube. SIC values lower than 4.0 should be considered as an insufficient amount of sample, and the sampling procedure must be repeated.
During amplification of biological samples that do not contain the human genomic DNA the Real-Time PR instrument should record the absence of exponential growth of the fluorescence level in the corresponding tube.
D. Diagnostics Characteristics
Number of samples (n) - 191; Diagnostic sensitivity (95% CI) - 99.3% (96.7-100%); Diagnostic specificity (95% Cl) - 99.9% (99.8-99.9%)