Genprice Gentaur
Monkeypox Virus Real Time PCR Kit | GEN-MPV-21
- SKU:
- GEN-MPV-21-GEN
- Availability:
- IN-STOCK
Description
Monkeypox Virus Real Time PCR Kit | GEN-MPV-21
Instruments:
Ⅰ:LightCycler 1.0 (Internal control can't be used for this system)
Ⅱ:LightCycler 2.0
Ⅲ:PE5700, MJ-Opticon & other single color systems
lesion exudate samples by using real time PCR systems.
2. Principle of Real-Time PCR
The principle of the real-time detection is based on the fluorogenic 5’nuclease assay. During the PCR reaction, the DNA polymerase cleaves the probe at the 5’ end and separates the reporter dye from the quencher dye only when the probe hybridizes to the target DNA. This cleavage results in the fluorescent signal generated by the cleaved reporter dye, which is monitored real-time by the PCR detection system. The PCR cycle at which an increase in the fluorescence signal is detected initially is proportional to the amount of the specific PCR product. Monitoring the fluorescence intensities in real-time allows the detection of the accumulating product without having to re-open the reaction tube after the amplification.
3. Product Description
Monkeypox virus is the virus that causes the disease monkeypox in both humans and animals.
Monkeypox virus is an Orthopoxvirus, a genus of the family Poxviridae that contains other viral species that target mammals. The virus is mainly found in tropical rainforest regions of central and West Africa. The primary route of infection is thought to be contact with the infected animals or their bodily fluids.The genome is not segmented and contains a single molecule of linear double-stranded DNA, 185000 nucleotides long.The Monkeypox Virus real time PCR Kit contains a specific ready-to-use system for the detection of the Monkeypox Virus through polymerase chain reaction (PCR) in the real-time PCR system. The master contains reagents and enzymes for the specific amplification of the Monkeypox Virus DNA. Fluorescence is emitted and measured by the real time systems´ optical unit during the PCR. The detection of amplified Monkeypox Virus DNA fragment is performed in fluorimeter channel FAM with the fluorescent quencher BHQ1. DNA extraction buffer is available in the kit and serum or lesion exudate samples are used for the extraction of the DNA. In addition, the kit contains a system to identify possible PCR inhibition by measuring the HEX/VIC/JOE fluorescence of the internal control (IC). An external positive control defined as 1×107copies/ml is supplied which allow the determination of the gene load. For further information.
4. Kit Contents

Note:
Analysis sensitivity depends on the sample volume, elution volume, nucleic acid extraction
methods and other factors .If you use the DNA extraction buffer in the kit, the analysis sensitivity is the same as it declares. However, when the sample volume is dozens or even hundreds of times greater than elution volume by some concentrating method, it can be much higher.
5. Storage
• All reagents should be stored at -20°C. Storage at +4°C is not recommended.
• All reagents can be used until the expiration date indicated on the kit label.
• Repeated thawing and freezing (> 3x) should be avoided, as this may reduce the sensitivity of the assay.
• Cool all reagents during the working steps.
• Reaction Mix should be stored in the dark.
6. Additionally Required Materials and Devices
• Biological cabinet
• Real time PCR system
• Desktop microcentrifuge for “eppendorf” type tubes (RCF max. 16,000 x g)
• Vortex mixer
• Real time PCR reaction tubes/plates
• Cryo-container
• Pipets (0.5 μl – 1000 μl)
• Sterile filter tips for micro pipets
• Sterile microtubes
• Disposable gloves, powderless
• Biohazard waste container
• Refrigerator and freezer
• Tube racks
7. Warnings and Precaution
Carefully read this instruction before starting the procedure.
• For in vitro diagnostic use only.
• This assay needs to be carried out by skilled personnel.
• Clinical samples should be regarded as potentially infectious materials and should be prepared in a laminar flow hood.
• This assay needs to be run according to Good Laboratory Practice.
• Do not use the kit after its expiration date.
• Avoid repeated thawing and freezing of the reagents, this may reduce the sensitivity of the test.
• Once the reagents have been thawed, vortex and centrifuge briefly the tubes before use.
• Prepare quickly the Reaction mix on ice or in the cooling block.
• Set up two separate working areas: 1) Isolation of the RNA/ DNA and 2) Amplification/
detection of amplification products.
• Pipets, vials and other working materials should not circulate among working units.
• Use always sterile pipette tips with filters.
• Wear separate coats and gloves in each area.
• Do not pipette by mouth. Do not eat, drink, smoke in laboratory.
• Avoid aerosols.
8. Sample Collection, Storage and transport
• Collect samples in sterile tubes.
• Specimens can be extracted immediately or frozen at -20°C to -80°C.
• Transportation of clinical specimens must comply with local regulations for the transport of
etiologic agents.
9. Procedure
9.1 DNA-Extraction
DNA extraction buffer is contained in the kit, please thaw the buffer thoroughly and spin down brieflyin the centrifuge before use. You may use your own extraction systems or commercial kits.
- Pipet 50μl sample(serum, or lesion exudates dissolved in 1ml saline )to a 0.5ml tube, add 50μl
DNA extraction buffer, close the tube and vortex for 10 seconds. Spin down briefly in a table
centrifuge. - Incubation the tube for 10 minutes at 100°C.
- Centrifuge the tube at 13000rpm for 10 minutes. The supernatant contains the DNA extracted and is used for PCR template.
9.2 Internal Control
It is necessary to add internal control (IC) in the reaction mix. Internal control (IC) allows the user to determine and control the possibility of PCR inhibition.
Add the internal control (IC) 1μl/rxn and the result will be shown in the HEX/VIC/JOE.
9.3 Quantitation
The kit can be used for quantitative or qualitative real-time PCR. A positive control (1×107copies/ml) is supplied in the kit.
For performance of quantitative real-time PCR, Standard dilutions must prepare first as follows.
Molecular Grade Water is used for dilution.
Dilution is not needed for performance of qualitative real-time PCR.
Take positive control (1×10^7copies/ml) as the starting high standard in the first tube. Respectively pipette 36ul of Molecular Grade Water into next three tubes. Do three dilutions as the following figures:
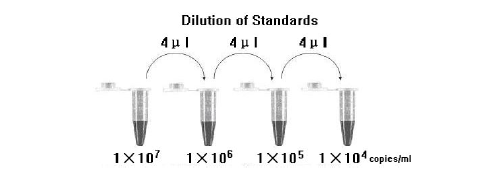
To generate a standard curve on the real-time system, all four dilution standards should be used and defined as standard with specification of the corresponding concentrations.
Attention:
A. Mix thoroughly before next transfer.
B. The positive control (1×107copies/ml) contains high concentration of the target DNA. Therefore, be
careful during the dilution in order to avoid contamination.
9.4 PCR Protocol
The Master Mix volume for each reaction should be pipetted as follows:
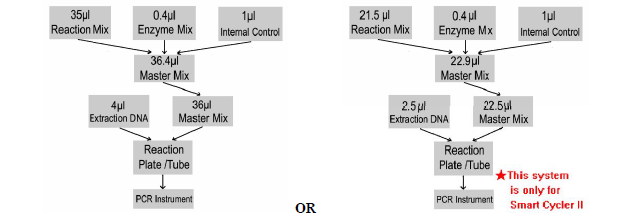
PCR system without HEX/VIC/JOE channel may be treated with 1μl Molecular Grade Water instead of 1μl IC.
1) The volumes of Reaction Mix and Enzyme Mix per reaction multiply with the number of samples, which includes the number of controls, standards, and sample prepared. Molecular Grade Water is used as the negative control. For reasons of unprecise pipetting, always add an extra virtual sample. Mix completely then spin down briefly in a centrifuge.
2) Pipet 36μl (22.5μl for SmartCyclerII) Master Mix with micropipets of sterile filter tips to each of
the real time PCR reaction plate/tubes. Separately add 4μl(2.5μl for SmartCyclerII) DNA sample,
positive and negative controls to different reaction plate/tubes. Immediately close the plate/tubes to avoid contamination.
3) Spin down briefly in order to collect the Master Mix in the bottom of the reaction tubes.
4) Perform the following protocol in the instrument:
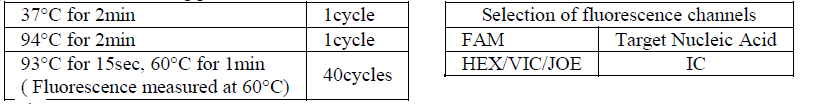
5) If you use ABI Prism® system, please choose “none” as passive reference and quencher.
10. Threshold setting: just above the maximum level of molecular grade water.
11. Calibration for quantitative detection: Input each concentration of standard controls at the end of run, and a standard curve will be automatically formed.
12. Quality control: Negative control, positive control, internal control and QS curve must be
performed correctly, otherwise the sample results is invalid.

13. Data Analysis and Interpretation
The following results are possible: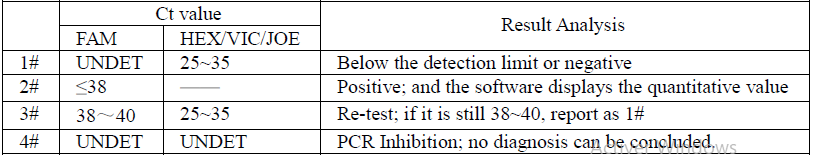
Additional Information
Nucleic Acid: |
ADN |
Packing (Tests/Kit): |
25 Reactions |
Lead time: |
3 Weeks |






