2
NATtrol Chlamydia trachomatis serotype D, External Run Control, Low
- SKU:
- 02-NATCT(D-UW3)-ERCL-GEN
- Availability:
- IN STOCK
Description
NATtrol Chlamydia trachomatis serotype D, External Run Control - Low- 6 X 1 mL
PRODUCT DESCRIPTION:
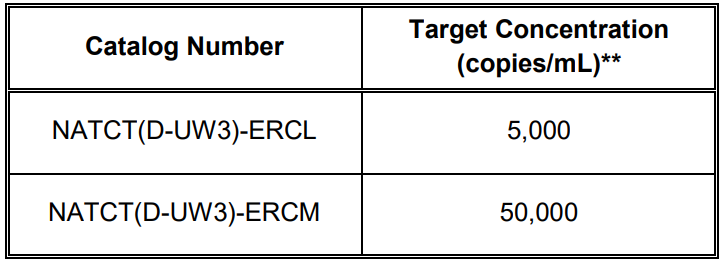
NATtrol™ Chlamydia trachomatis External Run Controls (NATCT(D-UW3)-ERCL and NATCT(D-UW3)-ERCM)* are formulated with purified, intact bacterial cells that have been chemically modified to render them non-infectious and refrigerator stable. Each control pack contains 6 x 1.0 mL vials of C. trachomatis NATtrol™ at target concentrations listed in Table 1. These controls are supplied in a purified protein matrix that mimics the composition of a true clinical specimen.
INTENDED USE:
- NATtrol™ Chlamydia trachomatis External Run Controls are designed to evaluate the performance of nucleic acid tests for determination of the presence of C. trachomatis DNA. NATCT(D-UW3)- ERCL and NATCT(D-UW3)-ERCM can also be used for validation of clinical assays, development of diagnostic tests and training of laboratory personnel.
- NATCT(D-UW3)-ERCL and NATCT(D-UW3)-ERCM contain intact organisms and should be run in a manner identical to that used for clinical specimens.
ETIOLOGIC STATUS/BIOHAZARD TESTING:
- NATtrol™ inactivation was carried out on the C. trachomatis stock used to formulate each control pack. The inactivation was verified by the absence of Chlamydial inclusion forming units (IFU).
- Purified protein matrix used in the manufacture of this product is treated with 0.09% sodium azide. It was manufactured from materials that have been tested and found non-reactive at the donor level for HIV-1/HIV-2 Antibody, HBsAg and HCV Antibody by FDA licensed donor screening test methods. All materials are also tested for HIV-1 and HCV by FDA approved Nucleic Acid Test (NAT) methods. Heat inactivated bovine based source materials used in the manufacture of this product meet applicable USDA requirements for abattoir sourced animals, traceability and country of origin. The materials were collected at USDA licensed establishments or legally imported from countries recognized by the USDA as negligible or controlled for risk for Bovine Spongiform Encephalopathy (BSE) and other exotic disease agents. Donor animals were inspected ante and post mortem at the abattoir as required by the USDA.
PRECAUTIONS:
- Although NATCT(D-UW3)-ERCL and NATCT(D[1]UW3)-ERCM contain inactivated organisms, they should be handled as if potentially infectious.
- Use Universal Precautions when handling this product.
- To avoid cross-contamination, use separate pipette tips for all reagents.
RECOMMENDED STORAGE:
- NATtrol™ Chlamydia trachomatis External Run Controls should be stored at 2-8°C.
INSTRUCTIONS FOR USE:
- Extract C. trachomatis DNA prior to use in downstream assays.