Description
NATtrol™ CT/NG External Run Controls are qualitative in vitro diagnostic external run controls intended to be used with molecular assays.
SUMMARY AND EXPLANATION:
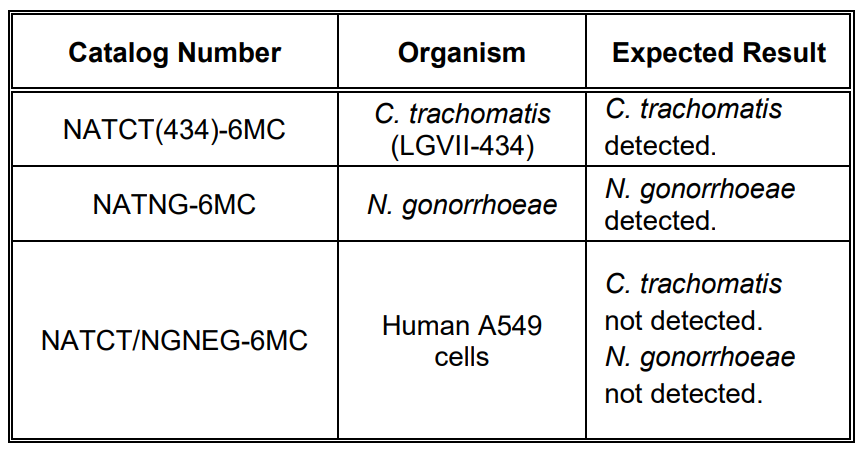
NATtrolTM Chlamydia trachomatis (CT) Positive Control and NATtrol™ Neisseria gonorrhoeae (NG) Positive Control contain purified, intact microorganisms that have been chemically modified to render them non-infectious and refrigerator stable.
NATtrol™ CT/NG Negative Control contains intact human A549 cells. The controls are formulated in a proprietary matrix.
PRINCIPLE:
Controls should be used according to assay manufacturer’s instructions. The routine use of external run controls enables
laboratories to monitor test variation, lot-to-lot test kit performance, operator variation, and can provide assistance in identifying random or systemic error.
MATERIALS SUPPLIED:
Each control pack is supplied separately and contains 6 x 1.0 mL vials NATtrolTM Chlamydia trachomatis (CT) Positive Control,
NATtrol™ Neisseria gonorrhoeae (NG) Positive Control, or NATtrol™ CT/NG Negative Control. Each control contains 0.09%
sodium azide.