Description
NATtrol Pneumonia Panel - Quantifiable Bacteria - 17 x 0.2 mL, 3 x 1.2 mL
PRODUCT DESCRIPTION:
NATtrol™ Pneumonia Verification Panels: NATtrol™ Pneumonia Panel
– Quantifiable Bacteria (NATPPQ-BIO)* and NATtrol™ Pneumonia Panel
– Atypical Bacteria & Viruses (NATPPA-BIO)* are formulated with purified,
intact virus particles and bacterial cells that have been chemically modified
to render them non-infectious and refrigerator stable. NATPPQ-BIO panel
contains 17 x 0.2 mL vials of bacterial NATtrol™ and 3 x 1.2 mL vials of
Negative Control as listed in Table 1. NATPPA-BIO panel contains 12 x
0.2mL vials of viral and bacterial NATtrol™ and 2 x 1.2mL vials of Negative
Control as listed in Table 2. The panels are supplied in proprietary matrix.
INTENDED USE:
• NATtrol™ Pneumonia Verification Panels are designed to
evaluate the performance of nucleic acid tests for determination
of the presence of viral and bacterial nucleic acids (from
organisms listed in Table 1). NATPPQ-BIO and NATPPA-BIO
enables laboratories to monitor test variation, lot-to-lot test kit
performance, operator variation and can provide assistance in
identifying random or systemic error.
WARNINGS AND PRECAUTIONS:
• NATtrol™ inactivation was carried out on microorganism stocks
used to formulate the panel members. The inactivation was
verified in a standard microbiological growth protocol.
• This panel contains inactivated microorganisms and materials of
human and animal origin. Safe practices suggest that the
controls be considered potentially infectious and to use Universal
Precautions when handling.
• Refer to CDC guidelines and local regulations for handling and
disposal.
• The matrix used in the manufacture of this product is treated with
0.09% sodium azide. It was manufactured from Human Serum
Albumin that have been tested and found to be non-reactive at
the donor level for HIV-1/HIV-2 Antibody, HBsAg and HCV
Antibody by FDA licensed donor screening test methods. All
materials are also tested for HIV-1 and HCV by FDA approved
Nucleic Acid Test (NAT) methods.
• Heat inactivated Fetal Bovine Serum used in the manufacture of
this product meet applicable USDA requirements for abattoir
sourced animals, traceability and country of origin. The materials
were collected at USDA licensed establishments or legally
imported from countries recognized by the USDA as negligible or
controlled for risk for Bovine Spongiform Encephalopathy (BSE)
and other exotic disease agents. Donor animals were inspected
ante and post mortem at the abattoir as required by the USDA.
• Do not use past the expiration date on the label.
• To avoid cross-contamination, use separate pipette tips for all
materials.
RECOMMENDED STORAGE:
• NATtrol™ Pneumonia Verification Panels should be stored at
2-8°C.
INSTRUCTIONS FOR USE:
• Mix vial vigorously for at least 5 secs.
• Process according to manufacturer’s instructions for sample to
result assays.
• Extract nucleic acid prior to use in downstream assays that are
not sample to result.
LIMITATION:
• FOR RESEARCH USE ONLY. NOT FOR USE IN
DIAGNOSTIC PROCEDURES
• Quality control materials should be used in accordance with local,
state, federal, and accreditation requirements.
• This product is not intended to replace the manufacturer’s controls
provided with the assay.
EXPECTED RESULTS:
• Each laboratory must evaluate the product and establish their own
acceptance criteria.
• This panel has been tested with the BioFire® Pneumonia Panel
assay and provides all expected results for the panel
members listed in Table 1.
• The tables shown below are for informational purposes only.
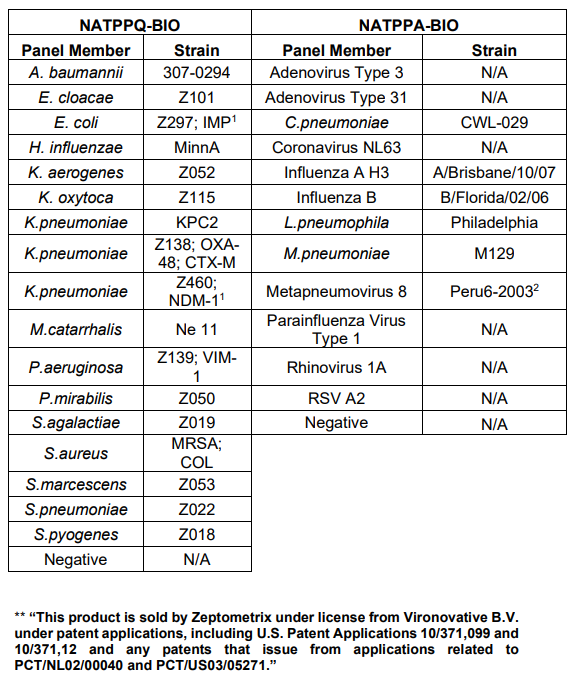
Table 1. Panel Members
This strain is sourced and used under license from the National Collection of Type Cultures
(NCTC®), Public Health England. 2 This product is sold by ZeptoMetrix under license from Vironovative B. V. under patent
applications, including U.S. Patent Applications 10/371,099 and 10/371,12 and any patents
that issue from applications related to PCT/NL02/00040 and PCT/US03/05271.