Description
PeriodontScreen RT-PCR | T01707-96-S is available for delivery
Real Time PCR test for detection and quantification of Porphyromonas endodontalis, Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Treponema denticola, Fusobacterium nucleatum, Prevotella intermedia, Tannerella forsythia
Storage & Shipping :
The kit can be shipped at 2-8°C for 3-4 days but should be stored at 2-8°C and -20°C immediately on receipt.
PeriodontScreen RT-PCR (CE) T01707-96-S DataSheet
INTRODUCTION
According to the World Health Organization from 80 to 100% of the adult population in different age groups, suffer inflammatory periodontal diseases. Periodontal diseases along with dental caries and its complications are the most common dental diseases. It is now established that in patients older than 40 years, the cause of tooth loss is more periodontal disease than tooth decay.
The etiology of periodontal disease.
One of the main causes of the development of periodontal diseases are specific bacterial agents. Additional risk factors include genetic predisposition of the immune system, poor oral hygiene, smoking, systemic diseases and stress (Fig. 1).
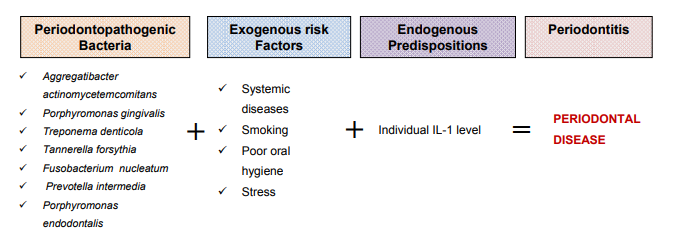
Figure 1: Periodontal disease risk factors
Microbial factor, being one of the most powerful etiologic agents, causes various clinical manifestations of periodontal disease. A significant role in pathogenesis of periodontitis have the composition and types of microorganisms in dental plaque, its volume, time of exposition of the areas of gingival and periodontal tissues. These factors, according to most researchers, explain the development of inflammatory changes in the periodontium with different clinical manifestations.
The infectious nature of periodontal disease
Periodontal diseases are a diverse group of infectious diseases of periodontal tissues with different specific pathogens, which are non-clostridial anaerobic bacteria. According to the WHO classification, they are grouped in the so-called Periodontopathogenic Bacteria (PB) [Fig. 2]. It is now established that this group of pathogens is a major cause of progressive periodontal disease. This leads to the need for specific methods of diagnosis and treatment of identified bacteria in chronic-progressive-resistant and aggressive periodontal disease. Purely mechanical treatment is generally ineffective, particularly when PB are present in oral cavity and in this case it should be used antibacterial therapy. A necessary condition for choosing the appropriate antimicrobial agent is the knowledge of the bacterial spectrum and the concentration of present microorganisms.
Biological markers of periodontal disease
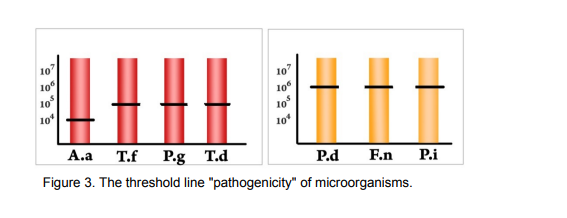
Periodontopathogenic Bacteria are in divided in high and medium risk of developing the disease.

Figure 2: List of high and medium risk Periodontopathogenic Bacteria
All microorganisms detected by "PeriodontScreen Real-TM" panel have different pathogenic potential. A positive test result is when the concentration of the desired periodontopathogens is above clinically significant titer.
The physician must evaluate the total potential pathogenic microflora, depending on the type of microorganism and its concentration according to the data presented in the figure below. Identification of microorganisms and their concentration allows not only to choose the treatment regimen, but also to carry out its control.
INTENDED USE
PeriodontScreen Real-TM PCR kit is an in vitro nucleic acid amplification test intended for quantitative detection of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Porphyromonas endodontalis, Treponema denticola, Fusobacterium nucleatum, Prevotella intermedia, Tannerella forsythia. This kit uses endogenous Internal Control which is present in each reaction tube containing PCR mastermix. The endogenous IC detects human genomic DNA sequence which must always be present in each extracted sample.
This approach allows to monitor not only possible reaction inhibition but also:
- correct collection of clinical specimens;
- effectiveness of sample preparation;
- errors in the analysis (sample not added in the amplification mixture);
PRINCIPLE OF ASSAY
PeriodontScreen Real-TM is a QUANTITATIVE test that allow the detection by Real Time PCR based on the amplification of the genome specific region using specific primers. In Real Time PCR the amplified product is detected using fluorescent dyes. These dyes are linked to oligonucleotide probes that bind specifically to the amplified product. The real-time monitoring of the fluorescence intensities during the reaction allows the detection of accumulating product without re-opening of the reaction tubes after the PCR run.






