AMDX
Super-ARMS® EGFR Mutation Detection Kit | 8.01.20213X012
- SKU:
- AMDX-AM-8.01.20213X012-GEN
- Availability:
- IN STOCK
Description
Super-ARMS® EGFR Mutation Detection Kit | 8.01.20213X012 | AmoyDx
NSCLC tissue testing has been applied to EGFR mutation detection for years. However, up to 25% of patients with advanced or metastatic NSCLC do not have an available or sufficient tumor tissue sample for this method of testing. More and more dynamic monitoring demand for EGFR mutation status appears. When tissue sample is not a reliable or realistic option, the ctDNA (circulating tumor DNA) obtained from blood sample can be used for the assessment of EGFR mutation status.
Super-ARMS® EGFR kit is a highly sensitive, real-time PCR-based test which is designed to identify ctDNA EGFR mutation in plasma samples for patients with advanced or metastatic NSCLC. The kit adopts innovative Super-ARMS® technology which is upgraded from ADx-ARMS technology. Its optimized reaction system can rapidly and accurately detect low percentage of mutant ctDNA in a background of wild-type DNA.
Technological Principles:
The Super-ARMS® EGFR mutation kit enables detection of the following 42 EGFR mutations:
• Exon 18: G719C, G719A, G719S
• Exon 19: 29 deletions
• Exon 20: T790M, S768I, 6 insertions
• Exon 21: L858R, L861Q
Testing Procedure
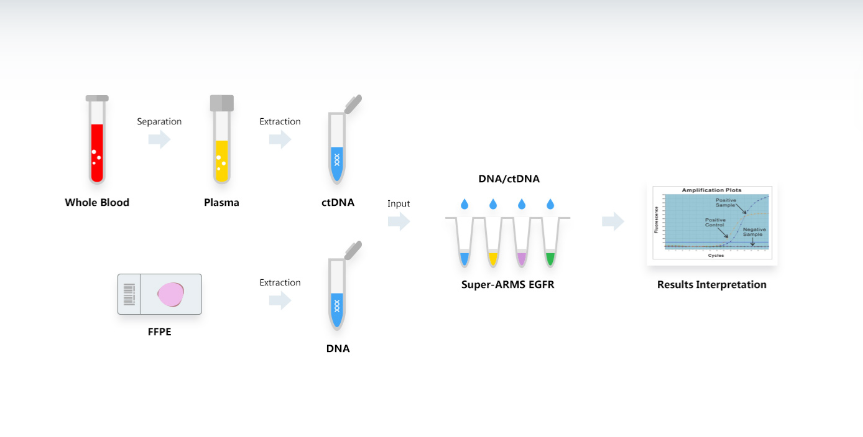
Additional Information
Size: |
12 Tests/Kit |
Type: |
Bulk |
Group I: |
Stratagene Mx3000P™, ABI 7500, LightCycler480 II, cobas® z480, SLAN-96S, QuantStudio 5, QuantStudio 12 Flex |
Group II: |
Rotor-Gene Q (36 wells) |






