Description
Monkeypox Virus Ag Rapid Test | GEN-MPV-01
1. INTENDED USE
The product is a lateral flow chromatographic immunoassay for the qualitative detection of monkeypox virus antigen in human whole blood, serum. plasma or rash exudate The kit is intended for professional use only.
2. INTRODUCTION
Monkypox virus„ first identified in monkeys in 1958, is a double-stranded DNA virus belonging to the same farnilv as smallpox virus, but is considerably less virulent than smallpox. Monkypox virus is a rare viral zoonosis (a virus transmitted from an to human). The main mode of transmission is dose contact including close contact with respiratory secretions. Skin lesions or contaminated objects of an infected person, and mother-to-child transmission and sexual transmission. Initial symptoms of monkeypox infection in human include fever, headache. muscle aches. back pain and swollen lymph nodes. which can progress to a widespread rash on the face and body.
3. PRINCIPLE
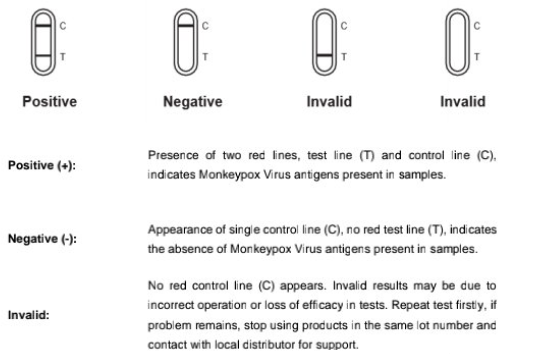
This product uses dual antibody sandwich method. During the test. a specimen is dropped into the sample well, and then the specimen is superimposed under the capillary effect. If the specimen contains monkeypox virus, A color band appears in the test area (T) indicate a positive result for monkeypox virus. If the specimen does not contain the corresponding substance to be tested_ there will be no color bands in the test area (T). and the result will be negative. A color band appears in the quality control area (C) regardless of whether the Corresponding substance to be tested is present in the specimen_ The color band in the quality Control area is. the standard to determine whether there are enough Specimens and whether the chromatographic process is normal, and also serves as the intema.1 control standard of the test.
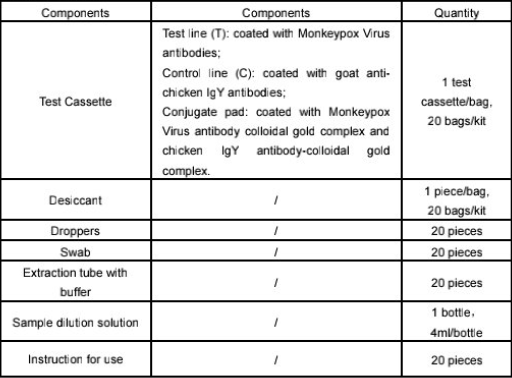
4. Components
5. Storage And Expiration Date
- Test should be stored at 2-30*C in dark and dry place for 24 months. DO NOT freeze the test;
- Test cassette is recommended to be used within 0.5 hour after opening the pouch; If the temperature is higher than 30'C or in high humidity environment, it should be to use immediately.
- Refer to the labe's to check the production date and expiry date of the kit.
6. MATERIALS NEEDED BUT NOT SUPPLIED
- Timer
7. SPECIMEN COLLECTION AND PREPARATION
Consider any materials of human origin as infectious and handle them with standard bio- safety procedures.
7.1 Plasma:
1. Collect blood specimen into a lavender, blue or green top colection tube (containing EDTA citrate or hepann, respective y, in Vacutainer®) by venipuncture.
2. Separate the p'asma by centrifugat on.
3. Carefuly withdraw the plasma into a new pre-labe ed tube. 7.2 Serum: 1. Collect blood specimen into a red top collection tube (containing no anticoagulants in Vacutainer8) by venipurcture.
2. Allow the blood to cot. 3. Separate the serum by centnifugation.
4. Carefuly wthdraw the serum into a new pre-labeled tube. Test specimens as soon as possibe after co lecting. Store specmens at 2'C-8*C if not tested immed ate y Specmens can be stored at 2'C -8*C for up to 5 days. The specimens should be frozen at -20°C for a longer storage. Avoid multiple freeze-thaw cycles. Prior to testing, bring frozen specimens to room temperature slowly and mix gently. Specimens containing visible particulate matter should be clarified by centrifugation before testing. Do not use samples demonstrating gross lipernia, gross hemolysis or turbidity in order to avoid interference on a result interpretation.
7.3 Blood:
Drops of whole blood can be obtained by either finger tip puncture or venipuncture. Whole blood specimens should be stored in refrigeration (2-8*C), if not tested immediately, the specimens must be tested within 24 hours of collection.
7.4 Rash Exudate:
1. Wipe the rash exudate with a sterile swab;
2. Insert the swab into the extraction tube with the buffer,
3. Roll the swab at least 6 times while pressing the head against the bottom and side of the extraction tube.
4. Leave the swab in the extraction tube for 1 minute.
5. Squeeze the tube several times with fingers from outside of the tube to immerse the swab. Remove the swab. The extracted solut on will be used as a test sample.
8. TEST PROCEDURE
Read the instructions thoroughly before testing and bring the cassette and specimen to room temperature.
8.1 For Plasma/Serum/Whole Blood:
1. Bring the specimen and test components to room temperature if refrigerated or frozen. Once thawed, mix the specimen weil prior to performing the assay.
2. When ready to test, open the pouch at the notch and remove the device. Place the test device on a cean and flat surface.
3. Fill the plastic dropper with the specimen. Holding the dropper vertically. dispense 1 drop of serum/plasma (about 30-45 uL) or 1 drop of whole blood (about 40-50 uL) into the sample well, making sure there are no air bubbles
4. Immediately add 1 drop (about 35-50 pL) of sample divent with the bottle positioned vertically. 5. Wait for colored line(s) to appear. Interpret the test resu'ts in 15 minutes. Do not read results after 20 minutes.
8.2 For Rash Exudate:
1. Remove test Cassette from the sealed pouch just prior to the testing and lay flat on workbench.
2. Insert a nozzle with fiter into the sample extraction tube tightly.
3. Reverse the sample extraction tube, and add 3 drops sample by squeezing the extracted solution tube into the sample window.
4. Wait for colored line(s) to appear. Interpret the test results in 15 minutes. Do not read results after 20 minutes.
9. Interpretation of Resultas